- Immunology /
- Services /
- c-KIT MCT assay
c-KIT Mutation analysis in canine Mast cell tumours (c-KIT MCT Assay)
Revisiting the correlation of c-kit mutation status and treatment decisions in canine Mast Cell Tumors
Background: Mast cell tumors (MCTs) are the most frequent skin tumors in dogs, with an incidence of 16-21% of all tumors. Mutations in the proto-oncogene c-Kit, which encodes for the transmembrane stem cell factor receptor on the mast cells surface, induce constitutive receptor activation.
Objective: Up to 50% of canine MCTs exhibit internal tandem duplications (ITDs) in either exon 8 or 11 promoting cell growth and survival. We aimed to establish an indication for the treatment with tyrosine kinase inhibitors, based on the c-Kit mutation status of the canine MCT patients.
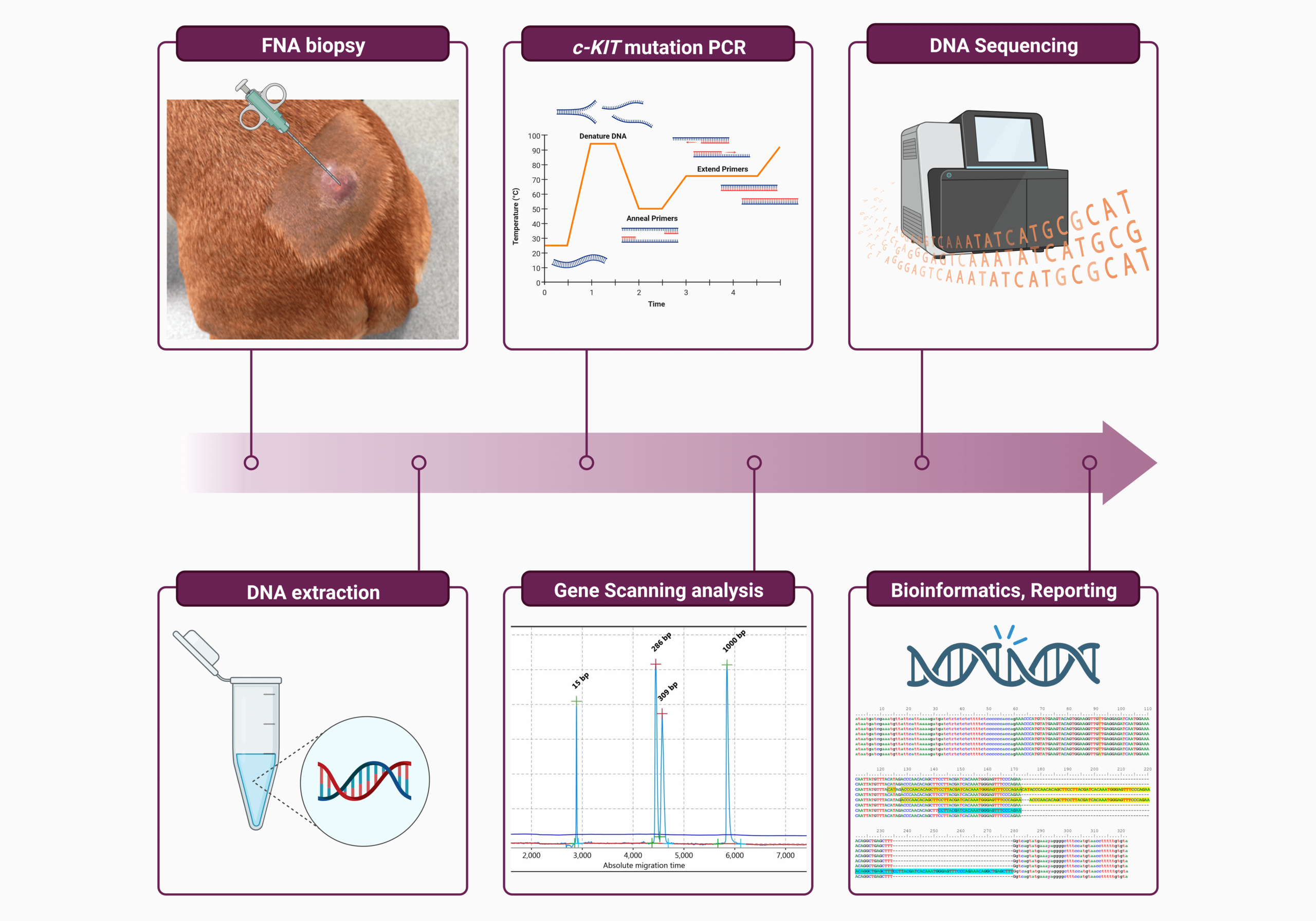
Methods: In 53 histopathological confirmed MCT dogs, the c-Kit exons 8, 9, 11, 13, 14 and 17 were investigated by isolating genomic DNA from remnant diagnostic material and subsequent sequence comparisons to healthy and malignant reference material.
Results: For exon 8, one ITD was found, and only twelve patients showed ITDs in exon 11. For the latter exon, additional eight dogs exhibited the same silent mutation, not been detected in the reference material. Interestingly, in one patient, the high-grade (Kiupel)/ grade III (Patnaik) tumor stage did correlate with an amino acid exchange V563D (T>A1688). This driving mutation in human gastrointestinal stromal tumors could therefore also be an activating mutation in canine mast cell tumors.
Conclusions: For c-Kit mutation analysis, a time- and cost-efficient work routine was established. The low ITD frequency in exons 8 and 11 together with the absence of other know mutations suggest that the c-Kit mutation status alone is not sufficient to make treatment decisions.
Authors: Sabine E. Hammer1, Amina Paquay1, Giovanni De Zottis1, Viktoria Klingerstorff1, Andrea Fuchs-Baumgartinger2, Nicole Luckschander-Zeller3, Ilse Schwendenwein4, Barbara C. Rütgen4
Affiliations: 1Institute of Immunology, 2Institute of Pathology and 4Clinical Pathology Unit, Department of Pathobiology; 3Clinic for Internal Medicine, Department/ Hospital for Companion Animals and Horses; 1-4University of Veterinary Medicine Vienna, Austria.
Reference: Accepted abstract for a platform presentation at the American College of Veterinary Pathologists (ACVP) and the American Society for Veterinary Clinical Pathology (ASVCP) Annual Meeting, November 12-15, 2022, in Boston, Massachusetts, during a Focused Scientific Session.
Selected References
- Paper 1
- Paper 2
- Paper 3
Sample Submission
Samples have to be shipped to the Central Laboratory (Clinical Pathology Unit) together with the sample submission form.
Detailed information about sample submission can be found by following this LINK.